Corcept Therapeutics to Start Phase 3 Trial of Relacorilant Plus Nab-Paclitaxel in Patients With Platinum-Resistant Ovarian Cancer
“We are excited to launch our pivotal Phase 3 trial, which we have named ROSELLA,” said
“The ROSELLA trial design closely tracks the design of our successful Phase 2 study, with adjustments that emerged from constructive conversations with the FDA and leading clinicians from the
ROSELLA has a planned enrollment of 360 women, randomized 1:1 to receive either relacorilant plus nab-paclitaxel or nab-paclitaxel monotherapy. The primary endpoint will be progression free survival, with overall survival as a key secondary endpoint.
All patients will have received prior bevacizumab therapy, which is the current standard of care in
Corcept plans to start ROSELLA by the end of this month.
In Corcept’s Phase 2 trial, women who met the entry criteria for ROSELLA and received relacorilant exhibited substantial benefit, without increasing the frequency or severity of adverse events. Results for patients who received relacorilant at the time they received nab-paclitaxel – the “intermittent” dosing regimen that will be used in ROSELLA – are set forth in Table 1.
| Phase 2 Trial Results (ROSELLA Patient Population) | |||
| Relacorilant + Nab-paclitaxel n=25 |
Comparator n=31 |
(95% CI) |
|
| Progression-Free Survival (median) |
7.3 months | 3.7 months | 0.40 (0.21, 0.77); p = 0.005 |
| Duration of Response (median) |
5.6 months | 3.1 months | 0.29 (0.09, 0.99); p = 0.016 |
| Overall Survival (median) |
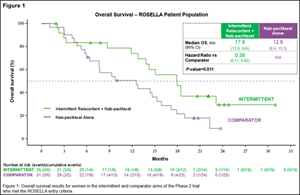
17.9 months | 12.6 months | 0.38 (0.17, 0.82); p = 0.011 |
| Table 1: Results for women in the intermittent and comparator arms of the Phase 2 trial who met the ROSELLA entry criteria. | |||
The Kaplan-Meyer curve showing the survival benefit experienced by women in the Phase 2 trial’s intermittent arm and who met the ROSELLA entry criteria is set forth in Figure 1.
ASCO Oral Presentation
Results from the company's Phase 2 study in patients with recurrent, platinum-resistant ovarian cancer will be featured in an oral presentation at the ASCO Annual Meeting today. Presentation slides are available at www.corcept.com/research-pipeline/publications.
Presentation Title: “Overall survival data from a 3-arm, randomized, open-label, phase 2 study of relacorilant, a selective glucocorticoid receptor modulator, combined with nab-paclitaxel in patients with recurrent platinum-resistant ovarian cancer.”
Speaker: Dr.
Presentation Time / Location:
About Platinum-Resistant Ovarian Cancer
Ovarian cancer is the fifth most common cause of cancer death in women.1 Patients whose disease returns less than six months after receiving platinum-containing therapy are described as having “platinum-resistant” disease. In
About Corcept’s Oncology Programs
There is substantial evidence that cortisol activity at the glucocorticoid receptor (“GR”) reduces the efficacy of certain anti-cancer therapies and that modulating cortisol’s activity may help anti-cancer treatments achieve their intended effect.
Many types of solid tumors express the GR and are potential targets for cortisol modulation therapy. In some cancers, cortisol inhibits cellular apoptosis – the tumor-killing effect many treatments are meant to stimulate. In other cancers, cortisol activity promotes tumor growth. Cortisol also suppresses the body’s immune response; activating – not suppressing – the immune system is beneficial in fighting certain cancers.
Corcept is conducting clinical trials of its proprietary selective cortisol modulators in combination with three different anti-cancer treatments in patients with ovarian, adrenal and prostate cancers. Corcept’s first controlled study in oncology – relacorilant plus nab-paclitaxel for the treatment of patients with ovarian cancer – has demonstrated statistically significant and clinically meaningful results.
About Relacorilant
Relacorilant is a non-steroidal, selective glucocorticoid receptor modulator that does not bind to the body's other hormone receptors. Corcept is studying relacorilant in a variety of serious disorders, including ovarian and adrenal cancer and Cushing’s syndrome. Relacorilant is proprietary to Corcept and is protected by composition of matter and method of use patents, as well as orphan drug designation in
About
Corcept has discovered a large portfolio of proprietary compounds that selectively modulate the effects of cortisol and owns extensive
Forward Looking Statements
Statements in this press release, other than statements of historical fact, are forward-looking statements based on our current plans and expectations that are subject to risks and uncertainties that might cause our actual results to differ materially from those such statements express or imply. These risks and uncertainties include, but are not limited to, our ability to operate our business, conduct our clinical trials and achieve our other goals during the COVID-19 pandemic; risks related to the development of relacorilant and other product candidates, including their clinical attributes, regulatory approvals, mandates, oversight and other requirements; and the scope and protective power of our intellectual property. These and other risks are set forth in our
In this press release, forward-looking statements include those concerning the development of relacorilant as a treatment for ovarian cancer, including its clinical attributes, regulatory approvals, mandates and oversight, and other requirements; the potential for relacorilant plus nab-paclitaxel to become a standard of care for patients with recurrent platinum-resistant ovarian cancer; our planned Phase 3 trial, including its design and start date, results and the probability of its success as a registrational study. We disclaim any intention or duty to update forward-looking statements made in this press release.
CONTACT:
Investor Relations
ir@corcept.com
www.corcept.com
1
2 Clarivate | Decision Resources Group Ovarian Cancer Market Forecast Dashboard -
3 Therapeutic Advances in Medical Oncology (Luvero et al. 2014)
4
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/4642fad3-bdfc-43ed-b1e5-e99d42d5c8cc

Source: Corcept Therapeutics Incorporated